For many of us, the most memorable bits of school chemistry classes were lessons where we ignited metal salts over a Bunsen burner to produce brightly coloured flames, from the lilac of potassium to the distinctive red of lithium. Now a group of chemists from Harvard University have found a way of using these colourful flames to transmit coded information.
Working in the lab of legendary chemist George Whitesides, Samuel Thomas III has developed the 'infofuse', a strip of flammable paper patterned with metal salts. As the strip burns, the metals change the colour of the flames, creating coded pulses of light that can be used to send messages. It's a vibrant, visual equivalent of Morse code and as a test-run, they used their infofuses to transmit the message, "LOOK MOM NO ELECTRICITY".
Thomas sees the infofuses as the first step toward a lightweight, self-powered form of communication that doesn't involve any electronics to store or transmit information. "We're interested in the intersection of information and chemistry," says Thomas, who dubs his work as 'infochemistry'. "Cells communicate using chemical signals, and we are interested in bridging the gap between that sort of chemical communication and the digital communication that our technological infrastructure is built on."
DNA is the biological epitome of this concept. Through a chain of molecules, it encodes instructions for building proteins that is then transmitted in the form of RNA and translated by enzymes. Outside the realm of biology, similar systems don't exist. You could think of signal flares, smoke signals of even litmus tests as ways of transmitting information through chemistry, albeit simple and slow ones. The infofuse is more sophisticated.
It is made of a highly flammable material called nitrocellulose or 'flash paper'. It burns with a 1,000C flame that moves along the paper at a constant speed, producing very little smoke and leaving no ash. Codes are written on the paper using small spots of metal ions dotted along the fuse strip using either a small pipette or an inkjet printer. As the strip burns, the wavelengths and order of the flames carry messages.
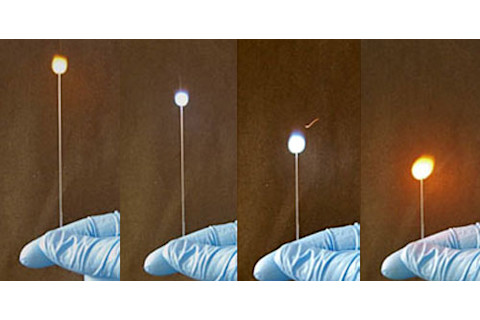
On its own, the nitrocellulose burns with a yellow-orange flame with strong signals that match those of sodium and potassium. The metal salts change the spectrum of the emitted light, making it shine brightly in the blue (copper), green (barium), yellow (sodium), red (lithium, strontium, calcium), or infrared (potassium, rubidium, and cesium) parts of the electromagnetic spectrum.
In the end, Thomas used the salts of just three metals - lithium, rubidium and caesium. He developed a code where every letter, every digit and four symbols (. ! ? @) were each represented by a unique combination of two consecutive pulses of light. In every pulse, each of the three emitters was either ablaze or not producing eight possible combinations of light. To ensure that the code is as unambiguous as possible, the combinations that were easiest to resolve were assigned to the most common letters (E, T and A). The combinations that could be most easily confused with one another were assigned to Q, Z and X.
As a test-run, Thomas managed to use the code to transmit the message "LOOK MOM NO ELECTRICITY". With a pulse frequency of 11Hz, the entire message took less than four seconds to transmit. Obviously, there's room for inaccuracy because the flame front moves, and small variations in the nitrocellulose strip can change the rate at which it burns and the intensities of the flames. But Thomas found that a bit of post-detection processing could easily adjust for these errors.
Fire-codes are certainly impressive, but to Thomas, they aren't just a matter of scientific curiosity. He says, "We envision that infofuses or similar materials could be used in situations where electricity is of limited availability or not available, or when carrying large quantities of batteries is difficult. Emergency situations are one area in which these could be useful: signal flares are ubiquitous, but do not transmit very much information. Infofuses could serve as signal flares that transmit specific information about the sender."
Obviously, the messages need to be detected by electronic equipment - either a special camera or a fibre optic cable linked to a spectrometer, a device that measures the intensities of different wavelengths of light. Thomas agrees, but he focuses on the many advantages provided by the signal's self-powered transmission.
It happens in every direction at one so that detectors can see it from any angle. At the moment, they can detect the fiery messages at a distance of 30 metres, even in bright daylight. The different wavelengths given off by the metal salts are very sharp and they stand out from their background to give the infofuse flames an exceptionally high signal-to-noise ratio. The burning is relatively inefficient, converting the energy released into light with just 1% of the efficiency of an LED but gram for gram, the burning nitrocellulose releases about 10 times more energy than an alkaline battery does.
And the technology is only in its infancy. There are many potential ways it could be developed. For a start, the messages on the infofuses can also be coded in different ways. The length of the metal spots on the strip determines how long the fiery pulse lasts for; the spacing between spots controls the time between the pulses; and the amount of salt that's added affects the intensity of the flame. Each of these variables could carry information. Thomas says, "We're expanding infofuses to last longer, transmit over longer distances and encode information more efficiently."
Reference: Thomas, S., Chiechi, R., LaFratta, C., Webb, M., Lee, A., Wiley, B., Zakin, M., Walt, D., & Whitesides, G. (2009). Infochemistry and infofuses for the chemical storage and transmission of coded information Proceedings of the National Academy of Sciences DOI: 10.1073/pnas.0902476106
More on technology:
DNA sculpture and origami - a meeting of art and nanotechnology
Plastic tubes and pipette tips leach chemicals that botch experiments