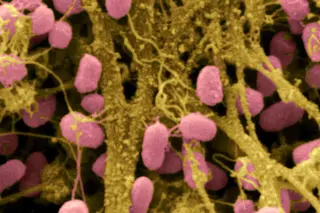
Touring a building with Jessica Green can be an unsettling experience. “We live nearly 90 percent of our lives indoors, but we know almost nothing about that environment,” she says as we push through the doors of the Lillis Complex, a four-story glass and concrete building on the University of Oregon campus in Eugene. “We don’t think about the wildlife in the air because we can’t see it. But it’s here.”
Inside the atrium, dozens of students bustle past on their way to class. Others chat with friends, send text messages, and order coffee from a small café. Meanwhile, in the air flowing around us and into our eyes, noses, mouths, and lungs, millions of microbes fight for survival. “Air isn’t empty,” Green continues. Not even close: A cubic meter of indoor air contains up to 10 million cells of bacteria. “Each one of us is shedding microbes from our ...