The costly tuberculosis (TB) drug bedaquiline, a lifesaver in more difficult cases, will be available in generic form to many low- and middle-income countries around the world, under a surprise agreement announced July 13, 2023. The accord was negotiated quietly between Johnson & Johnson and international group Stop TB Partnership, which provides TB drugs to lower income countries around the world.
Activists such as Doctors Without Borders and The Fault in Our Stars author John Green, who led a furor on Twitter, had worried the drug company would use patents in many countries to limit access.
But what is bedaquiline, and why has it become such a flashpoint?
The Largest Killer
Despite relatively low case numbers in the U.S. and other high-income countries, TB remains a major threat around the world, killing 1.5 million people a year. That has made it the deadliest infectious disease on the planet, a title eclipsed only briefly by COVID-19 during the worst of the pandemic.
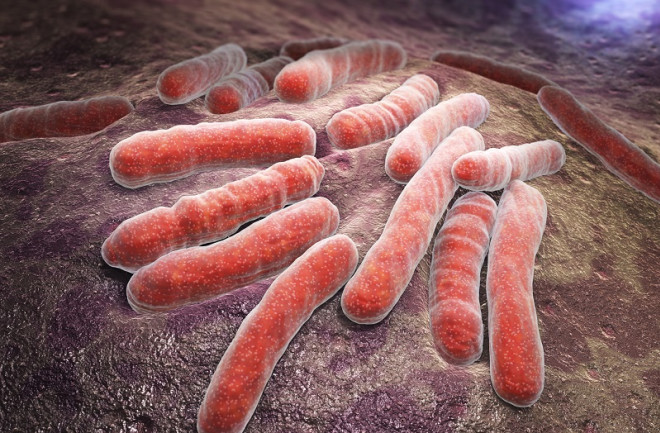
The respiratory infection is caused by Mycobacterium tuberculosis, which is known for its ability to outlast both the human immune system and an onslaught of TB drugs. The disease spreads through the air and can lurk there for several hours, waiting for someone to inhale it.
Not everyone who has TB has symptoms – a latent form of the disease also exists, but officials warn that it can later wake up and become the active form and infect others.
Read More: The "First Predators" Ruled a World Full of Bacteria
What Is Bedaquiline?
The recommended treatment for both latent and active TB is a long, arduous process that requires the patient to take multiple drugs every day for at least a few months. A person unlucky enough to contract a drug-resistant version of the disease may have to receive daily injections that carry heavier side effects.
Drug researchers have worked to develop a gentler oral medication that avoids drug resistance pitfalls while whittling down the length of time a person must take the drug. The poster child for these efforts, bedaquiline, received FDA approval in 2012 and represented the first novel mechanism to treat the disease in 40 years. It soon became the backbone of the World Health Organization’s (WHO) recommended regimes to fight TB.
Under international agreements, people in low- and middle-income countries pay less for bedaquiline, but a six-month supply still costs a few hundred U.S. dollars. Meanwhile, in wealthier countries such as the U.S., a similar course has cost as much as $30,000.
Read More: What Are Neglected Tropical Diseases?
How Was Bedaquiline Developed?
According to a 2021 presentation by one of its creators, Anil Koul – now a vice president at Johnson & Johnson – the researchers were initially impressed by TB’s ability to survive “in some of the most hostile environments.”
To bring the bacteria down a peg, they deployed a molecule to block one of its key helper enyzmes, protein kinase B, which showed promise at first. But when the researchers moved from in-vitro experiments to trials in mice, the results failed to show the chemical’s effectiveness against the disease.
Next they targeted ATP synthase, the pivotal enzyme that manufactures energy in the TB microbe, and early tests again went well. The resulting molecule seemed to be doing its job of flying into the complex, machine-like enzyme and shutting it down like a wrench thrown into an engine. Without energy, the bacteria ceased to function and eventually died.
“This was the magic of bedaquiline,” Koul said.
Read More: The History of the Lab Rat
The First Trials
The first tests with TB-infected mice showed great promise, but the first go-round with human subjects, a one-week study, went poorly. The new substance showed almost no efficacy against the infection.
“At that time, we thought about whether this was the end of the whole program,” Koul said, “and whether it makes sense to move further or not.”
The researchers later determined that the bacteria had simply gone inert and stopped multiplying, delaying the drug’s effectiveness until after 6 days. During the next, longer trial, which ran for two months, bedaquiline finally showed its striking efficacy and cut infection rates by about half.
Researchers later found that it had fewer issues with drug resistance, and it’s now used in cases of multi-drug-resistant TB and others labeled as extensively drug-resistant.
“It is extremely difficult to manage these patients,” Koul said.
Read More: The History of the Polio Vaccine