When 5-year-old Jonathan Alava complained about stomach pains one day in February 2012, his mother, Shinna, laid him on the couch and rubbed his tummy. Only the flu, she figured; another preschool bug. Then she felt the hard lump, immediately knowing it didn’t belong and dreading its meaning. Diagnosis: a malignant tumor 10 centimeters wide, the size of a small grapefruit.
For more than 10 months, the child’s journey followed the kind of dreary, double-edged protocol so familiar to cancer patients and their families. The blunt weapons against cancer tend to be both helpful and harmful, especially in children. First came relentless weeks of chemotherapy to reduce the mass — enveloped, in Jonathan’s case, in a tangle of blood vessels doctors dared not disrupt with surgical tools. Chemo is a toxic brew that destroys microscopic metastases, errant cancer cells that have spread throughout the body, along with many healthy ones. Vomiting became a regular part of Jonathan’s life.
Once the tumor was reduced to the size of a walnut, a surgeon could move in and remove the mass. But it had attached to Jonathan’s bladder, abdominal muscles and the iliac artery, a major thoroughfare for blood between the heart and the pelvis. Worse, after a biopsy, specialists spotted an even more disconcerting complication lurking at the edge of the pathology specimen: stray cancer cells. They would have to use radiation on the preschooler.
That’s when doctors say Jonathan got lucky. He had access to one of the most precise and powerful weapons in the modern anti-cancer arsenal, born out of regret after the bombings of Hiroshima and Nagasaki: a massive particle accelerator. Once exclusively the tool of physicists seeking to unravel mysteries smaller than the atom, the cyclotron has become the world’s most complicated and costly medical device. The machine, increasingly popular since it was introduced at a California medical center nearly 25 years ago, typically is as heavy as an airliner, surrounded by a shield of concrete and often housed in a building several stories tall. It delivers a straight, narrow beam of radiation that deposits most of its energy on the target tumor. Inflicting less collateral damage to surrounding organs and healthy tissues, it can minimize side effects and increase cure rates for some cancers — just what patients with small bodies like Jonathan need.
The accelerator achieves unparalleled precision by harnessing the power of protons, positively charged particles in the nucleus of an atom. These protons yank negatively charged electrons from their orbits around atoms within molecules. In the body, this process, known as ionization, strips away electrons from the DNA molecule, which houses the genetic instructions for a cell’s development and function. Without electrons, the DNA molecule falters, and the cell is obliterated or unable to proliferate. Unlike X-rays, which also ionize atoms, protons are heavier and contain more energy, allowing them to move in a straight, thin path. In this way, protons avoid indiscriminately mutilating a high proportion of healthy cells along with cancerous cells as photons do in other forms of radiation. The formidable energy of protons can be focused on the tumor itself and, amazingly, stopped at its far edge.
Yet the forces of commerce can eclipse the science. An investigation by Discover has found that the technology, which inspires an almost blinding awe even among sophisticated oncologists, likely is providing no benefit to many of the tens of thousands of patients steered to it. Despite complications sometimes more severe than with standard radiation therapy — and costs that are much higher for taxpayers, patients and insurers — proton beam therapy is heavily marketed for treatment of prostate cancer.
While enriching investors and giving medical centers and health providers a competitive edge, the popularity of treating prostate cancer with protons has had financial side effects, draining government coffers of millions of dollars. This is just one example of how profligate spending on treatments of marginal benefit contribute to runaway health care expenses. Across all health spending in America, an estimated $210 million is spent each year on unnecessary tests and procedures that do nothing to make patients healthier.
“One of the wonderful things about the U.S. health care system is that it’s rapidly rewarding, which leads to rapid innovation. But it does have a tendency toward excess,” says Harvard Medical School radiation oncologist Anthony Zietman, who was an early proton beam proponent before doubting its advantage in treating prostate cancer. He now is a co-investigator in the first randomized clinical trial comparing proton therapy with more conventional radiation — something he acknowledges should have happened before so much use.
“We rush into treatments before they are proved,” he says, referring to the wide array of pharmaceutical interventions and medical devices made available to U.S. consumers before thorough scientific scrutiny. “In some instances,” he adds, “proton therapy might be inferior to existing treatments.”
Exactly one research team has independently analyzed what prostate patients get for the money spent on proton treatment. Conclusion: not enough to justify the procedure. Over the past year, some regional insurers have begun to concur, refusing to pick up the tab for early treatment of prostate cancer. Even so, Medicare still subsidizes its use for prostate cancer, paying more than twice as much for proton treatment than conventional radiation — $50,000 vs. $20,000 — despite a distinct lack of proof that it’s superior.
That isn’t stopping doctors and medical centers from pushing proton therapy with the kind of enthusiasm once reserved for lucrative MRI machines or surgical robots — expensive technology that also has been overused. At price tags of $150 million or more, proton treatment centers can generate $50 million in revenue each year. Companies, governments and nonprofit medical centers are building or planning dozens of proton beam centers from Minnesota to China — in addition to the 42 already operating, 14 of which are in the U.S. It is simply modern medicine’s most audacious arms race.
Not that there are many complaints from pediatricians or parents of the relatively small number of children who develop uncommon cancers best treated with proton therapy. Proton therapy has been shown to be beneficial for certain uncommon ocular and brain cancers, and especially for treating children like Jonathan, whose developing tissues can be highly sensitive to stray radiation. In attacking the sarcoma in Jonathan’s belly, doctors could spare his bladder, rectum and pelvic bones, greatly reducing the risk of later disabilities.
But few cyclotrons would get built if it were just about the children. The same health care economics that generate revenues and hefty reimbursements for prostate cancer centers while siphoning millions from Medicare has essentially created subsidies that put a lifesaving technology within reach of the smallest patients. Unrestrained health markets are, in effect, taking from older men to pay for treatment of children like Jonathan.
Power of Redemption
Healing with protons had its origins in the effort to harness particles for once-unimaginable destruction — the Manhattan Project, which produced the first atomic bomb — and in the remorse of a scientist who helped create it. After the bombings of Hiroshima and Nagasaki, the application of nuclear knowledge to medicine served as an act of atonement for Robert Rathbun Wilson. Recruited by J. Robert Oppenheimer to head the project’s experimental division in 1944, Wilson, a young physicist at the University of California, Berkeley, was driven by the potential — he later called it rationalization — that the work would lead to a powerful new source of electricity, and a test detonation that would end the war.
Dreaming of Atoms
Robert Rathbun Wilson harbored dreams of helping create an intense and beneficial new source of energy from the most elemental building blocks of matter. As a boy in Wyoming, Wilson fiddled with vacuum tubes and pumps, and thrilled at the notion that one might likewise mix parts of the atom to “make the whole universe.”
During his childhood, scientists were swiftly uncovering more and more about the inner life of atoms, upending dogma going back to the ancient Greeks about the smallest components of all things. The atom, previously believed indivisible, could be artificially disintegrated, and chemical elements, long thought unalterable, could be metamorphosed.
In the first engineered “nuclear reaction,” in 1919, Ernest Rutherford produced oxygen from nitrogen by dislodging positively charged particles he called protons. “I have broken the machine and touched the ghost of matter,” Rutherford declared, giving birth to nuclear physics. Rutherford’s atomic model described a new world of worlds in which electrons orbited a nucleus like planets encircling the sun.
At the edge of this new frontier, Wilson’s professor and mentor at the University of California, Berkeley, Ernest O. Lawrence, invented the first crude cyclotron from shards of glass, wires and wax. It used magnetic fields to contain electrically charged particles in a narrow, spiraling path while an electric field accelerated them. “Atom-smashers,” as they became known, grew larger, more complex and powerful, pushing particles through the equivalent of a million volts — enough to create new elements.
Lawrence’s brother, John, a doctor at Yale, used one of the elements from his brother’s cyclotron, radioactive phosphorus, to examine metabolism in mice. Unexpectedly, mice injected with the radioactive phosphorus produced fewer white blood cells, and he theorized that the element suppressed production of bone marrow cells.
Wondering whether such radioactive isotopes might “perhaps attack cancer,” he injected the phosphorus from the cyclotron into mice with leukemia, a cancer of the bone marrow. A few weeks later, the irradiated mice were vigorous. The control group: dead. He had become the first to demonstrate the susceptibility of tumor cells to radiation.
Within months, the brothers irradiated people, including, in 1937, their mother, Gunda. She had inoperable uterine cancer, and specialists gave her three months to live. Though she grew violently ill, pleading for the treatments to stop, “this massive tumor just started evaporating,” John Lawrence recalled, according to an oral history kept at the University of California.
Gunda Lawrence lived for another 15 years, and radiation soon became a mainstay of cancer treatment. — K.E.
Instead, the first explosion proved an emotional turning point. “It literally dwarfed the great desert basin,” he later wrote. “My technical work was done, the race was run, and the full awful magnitude of what we had done came over me. I determined at that moment that having played a small role in bringing it about, I would go all out in helping to make it become a positive factor for humanity.” Returning to Berkeley was “a great relief … yet I still had a terrible load of guilt from having helped to build the bomb,” he acknowledged years later, in handwritten notes located by Discover at the archives of the Energy Department’s Fermilab.
In his earlier work at Berkeley, he gained intimate knowledge of the behavior of protons, using the cyclotron to measure their penetration into various materials. From that, he knew protons traveling through matter move in a straight line, depositing most of their energy in the final millimeters of their trajectory, when their speed slows. Whereas the photons used in conventional radiation therapy produced a kind of fog of small clumps of ionization, protons — 2,000 times heavier — left a dense and distinct line of ionization. This meant you could irradiate a strictly localized region within the body. He calculated the range of a beam the diameter of a needle.
“I worked out how much scattering and straggling would occur, and realized that much of the ionization would be deposited in a volume at the end of a narrow beam not much larger than the eraser on a pencil — and with very little exposure of the skin at the point of entry,” Wilson recalled in a draft for a speech. He thought of the beam as a heavy cannonball rolling through grass. Up against a single blade, it wouldn’t stop, but millions of collisions would bring it to a stop. The faster it was fired, the farther it would go.
In a 1946 paper in the journal Radiology, Wilson proposed that contemporary cyclotrons had almost become capable of energizing protons that could tackle deep-seated cancers. Depth and dose could be controlled. Initial speed of the charged particles would determine how deep in the body the particles would penetrate, while the intensity of the beam would determine the dose reaching the target. A more powerful dose could be exquisitely planned and directed anywhere in the body with more precision than X-rays. Finally there was a way to bend a particle to the purpose rather than blast a swath of sensitive tissue, as X-rays did.
“You can say it in one word: control,” explains James Slater, a proton pioneer who built the first hospital-based proton treatment center at Loma Linda University in Southern California. “The physician can control the proton. Nobody can control the photon.”
Wilson predicted a new cyclotron soon would produce “some real down-to-earth good.” He figured that, “in a few years, we’d be treating patients.”
His calculations proved better than his predictions. A decade passed before the first people were treated with protons, and even then only sporadically in research settings.
Mapping Treatment
The voyage of discovery is rarely a straight line, and despite Wilson’s insights, other pieces had to fall into place — some more serendipitous than scientific. For one thing, shooting a thin and powerful beam into the body requires the ability to see where it is going. At the time, a physician’s vision of the inside of a patient’s body was limited to shadowy X-ray images. Doctors crudely outlined boundaries for radiation exposure with wax crayons. They could only guess at depth and contours of a malignancy. “You had this precise beam — but you couldn’t see the tumor,” recalls Harvard radiation oncologist Zietman.
A handful of researchers refused to wait. Herman Suit, a radiation oncologist at Massachusetts General Hospital and one of Zietman’s mentors, had long been intrigued with the promise of curing people without opening them up. He had used cloned mice with identical tumors to determine ideal radiation doses before turning to protons. The implications of Wilson’s paper and his own studies on mice were “anything but subtle,” he says. A tumor could be irradiated with protons at higher doses than other radiation, with much less damage to normal tissue.
Suit invited himself to a cyclotron at Harvard, and in 1973 he persuaded its operators to let him use it on patients five days a week. Eventually he was treating many different kinds of cancers all over the body.
Suit recruited high-energy physicist Michael Goitein to Mass General and had him map out how protons interact with matter. That kind of information was too general, however, and the team knew they needed a unique map for each patient. At first, this required complicated mathematical calculations and large mainframe computers. Working from more than a dozen X-ray images to piece together a full image of the tumor and organs of the body, Goitein had to make thousands of calculations for each patient to figure out the angle, strength and penetration of the beam. Typically it took many hours of painstaking work. The task was prone to error and “devilishly hard,” Goitein says. “So I searched for technology that would make it easier.”
Building Boom
A Swiftly Growing Industry
Forty-two facilities are in use worldwide
In the U.S., 14 proton beam centers are operating, and another nine are being built. One of the largest is a $225 million facility at Hampton University in Virginia that lacks a medical school and plans to treat more than 2,000 patients per year with breast, lung, prostate, pediatric and other cancers.
The Mayo Clinic is spending more than $370 million, one-third from a wealthy donor, to bring accelerators to centers in Minnesota and Arizona.
Fortunately for Goitein and Suit, such technology was emerging: computed axial tomography (CAT) scanners, which could produce cross-sectional images, or “slices,” of the inside of the body. Computer graphics displays common to the first video games soon improved the task of visualizing the inside of the body and planning treatment. Like a pilot in a simulator, an oncologist could see the beam’s vector in virtual reality. By 1980, the team could afford a computer the size of several refrigerators with enough memory to handle the calculations needed to reconstruct the image.
With multidimensional vision, computing power and the Harvard cyclotron, the Mass General team could increase accuracy and design detailed treatment plans. “It was amazing stuff they were able to do clinically,” says Zietman, then a young doctor chosen by Suit for a fellowship at Mass General. “Herman Suit drove it and took it to the next level, and Goitein was the father of 3-D radiation planning. They took what Wilson had only talked about and put it to use. The proton beam wasn’t the big step forward; it was the understanding of how to treat in three dimensions.”
Yet conventional attitudes persisted. “There was a dogma in radiation oncology that a higher dose would be super lethal,” recalls Suit. Top radiation oncologists advised Suit to go back and review the standard protocol.
More than convention was at play. “The reason for resistance wasn’t science. It was the medical business,” says Suit. “If [radiation oncologists] were to say protons are better, and they didn’t have their own facility for it … they’d have to refer patients somewhere else. You’d be shipping out paying customers. It amounted to the fear of losing business.”
So cancer patients continued to endure photons.
James Slater, a young resident at a Los Angeles hospital during the 1960s, was horrified that radiation acted like a machete rather than as a scalpel. It just wasn’t precise enough. “We were making some patients too sick,” he recalled. “They dreaded it. I got to dread it myself.” He considered switching specialties, but then he decided he’d rather improve the specialty he was in.
By 1985, after years of taking patients to the research cyclotrons and seeing many of them benefit, Slater sought to bring the treatment to patients. He would build a proton accelerator inside a hospital. It opened at Loma Linda University in 1990, and the timing was good. A new screening tool for prostate cancer, the prostate-specific antigen test, enabled doctors to detect it earlier in more men, and patients were flocking to hospitals for low doses of conventional photon radiation. But the test also revealed that the standard radiation treatment wasn’t wiping out the cancer.
Studies on tumors in other parts of the body showed that protons might safely be used at higher doses than photons, so some radiation oncologists decided to give protons a try. In 1995, Zietman began a key study with Slater’s son, Jerry, at Loma Linda and Mass General comparing the standard dose of proton and photon treatment for prostate cancer with a higher dose. The first results, published in 2005, suggested men receiving the higher dose were more likely to be cured. “I imagined if proton beam [therapy] proved to be superior,” says Zietman, “nothing else could come close.”
That was what he thought then.
Are Protons Really Better?
At first, there seemed to be nothing to worry about. A handful of new proton beam treatment centers appeared to replicate successes at Loma Linda and Mass General, and they were meeting a worrisome need. While other cancers can be more lethal, prostate cancer is among the most feared when it strikes. It was, and still is, the most common malignancy in men, with 233,000 new diagnoses expected this year, and 29,480 deaths. Men took “radiation vacations” — proton treatment in the morning, sightseeing or even a round of golf in the afternoon. Referrals swelled, and the Internet helped popularize the procedure.
Through the 1990s, production of accelerators for medical treatment went commercial. The Belgian company Ion Beam Applications entered the market with an off-the-shelf prototype at Mass General in 1994. It cost far more than Mass General’s $46 million tab, but IBA swallowed the expense, gaining a foothold in the market, credibility and, soon enough, rising share value.
The popularity and cost of protons blindsided the federal government. Medicare in 2000 allowed proton treatment, on a case-by-case basis, for “conditions of possible benefit” — usually prostate cancer — never suspecting the number of treatment centers would grow. From 2006 to 2009, the number of beneficiaries doubled, and Medicare’s annual tab reached $27 million. As of March 2013, more than 105,743 patients have been treated around the world with protons — 17,829 of them at Loma Linda alone, according to the Particle Therapy Cooperative Group, which tracks usage.
As patients and revenues improved, so did competing technology and data on how patients fared in the years after treatment. Conventional radiation with photons gave way to intensity-modulated radiation therapy, or IMRT, in which more precise beams of photons could be moved dozens or hundreds of times with varying intensities, attacking tumors in three dimensions with safer high doses.
As data on proton treatments continued to dribble in, it was hard for Zietman to accept the implications of his own studies, which tracked patients’ progress. Their quality of life had not improved. They had not escaped the gastrointestinal issues, rectal bleeding, incontinence and impotence associated with other forms of radiation such as IMRT. One case still haunts Zietman — a patient with bleeding that couldn’t be stopped, the complications cascading until the patient was dependent on transfusions. The realization that something was amiss had been in the background. “I had a creeping sense of concern and despair,” he says.
Eventually, “the data was staring me in the face: Protons weren’t any better. They were being sold as if they had zero side effects.”
But they did have side effects.
In June 2011, a study of radiation therapy for prostate cancer found that patients receiving conventional radiation experienced fewer gastrointestinal problems than a similar group exposed to proton beams. In 2012, a separate study of 1,600 patients from 2002 through 2007 found that men treated for prostate cancer with proton beam therapy experienced more bowel problems, such as bleeding and blockages, than those treated with the more common IMRT. “We did not find a significant benefit in patient outcomes,” its lead author, University of North Carolina radiation oncologist Ronald Chen, told Discover. “Our results call for additional comparative effectiveness research.”
Zietman realized an experiment was the only way to settle the matter. In 2011, he helped lead the first randomized trial comparing IMRT with equivalent doses of protons. The first results aren’t expected for several years.
The industry, its backers and some clinicians say the trial is a waste of time and money — and unethical. Goitein, the physicist who helped bring 3-D to proton therapy, challenges the wisdom of subjecting half of the study’s 400 test subjects to IMRT instead of what he is convinced is a better therapy.
“Most people who do proton therapy believe physician and biological arguments are compelling enough to make it impossible to say to a patient with a straight face, ‘I think it is a toss-up,’ ” Goitein says. “People who advocate trials are ignoring the physical evidence.”
Zietman counters that good intentions and business imperatives sometimes blind scientists to the truth about risks and effectiveness. “It’s amazing how many people drank the Kool-Aid,” he says. “When you are enthralled to an investment company or have opened a center at enormous expense and must treat hundreds of patients, you kind of have to believe. You become an evangelist for a cause.”
A Weapon of Precision
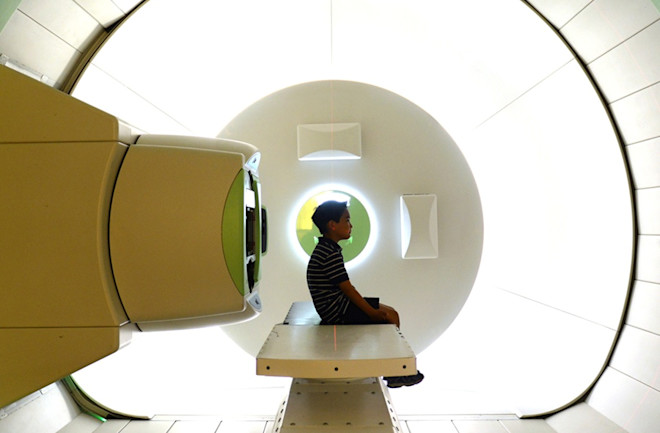
Each day for 20 days, Shinna and Jonathan Alava followed a new routine. Jonathan even began looking forward to the one-hour drive to the Children’s Hospital of Philadelphia from their home just south of Princeton, N.J. He enjoyed playing the hospital’s Wii video games until he fell into the kind of sleep induced by general anesthesia. Hospital staff wheeled him into a room on a gurney, taped his eyes shut, strapped him onto a treatment table, then directed the machine to aim a nozzle at him, unleashing the beam of energized subatomic particles at his pelvis.
The underground steel cyclotron, weighing 220 tons, is positioned, along with five gantries three stories tall, within a giant bunker the length of a football field beneath the bustling streets of West Philadelphia. Hydrogen gas is injected into the cyclotron, where static electricity separates protons from hydrogen molecules. Then massive magnets coerce the thin beam of protons into circular orbits as they accelerate toward the speed of light at 100,000 miles per second. Concrete walls, 6 to 10 feet thick, shield patients and personnel from dangerous neutrons released from the interactions of the proton beam.
Inside the University of Pennsylvania’s Roberts Proton Therapy Center, radiation oncologists sit in front of colorful, detailed images of patients’ bodies, clicking mice and tapping keyboards. Once the program is ready, the therapy team springs into action, and a traffic controller of sorts, positioned near the accelerator, observes animation of the beam as it moves through 100 yards of piping along five possible pathways to treatment rooms and, finally, through a small nozzle toward the patient.
The smallest cough or twitch can undermine the precision; moving even a centimeter can undo weeks of a radiation oncologist’s preparations and calculations, dispatching the beam in the wrong direction. This is why patients are anesthetized and strapped down. Jonathan’s treatment room, at 80,000 cubic feet, is 20 times the size of a typical CT or MRI room. The motorized gantry is 33 feet high and rotates around the patient to optimize the beam angle. Jonathan looks absolutely dwarfed and vulnerable.
For three weeks beforehand, Christine Hill-Kayser, the child’s radiation oncologist, worked out with colleagues just how strong a dose to deliver, and where in his pelvic area to direct the beam. Their computer screens filled with vivid colors as they calculated and checked their work, plotting targets and designating no-radiation zones. Jonathan’s pelvic bones flashed white — areas to avoid — while the intended trajectory for the protons glowed green and blue. The space where the tumor once mushroomed had a purple perimeter. Hill-Kayser circled the target area — only slightly larger than where the tumor had been — in bright yellow.
Although radiation oncologists can visualize the body far better than those who used wax crayons on X-ray scans, they still face thorny choices. Irradiating the area too far beyond the tumor’s edge can destroy healthy cells and sensitive tissues. “It can be very difficult finding that balance, that line between harm and benefit,” Hill-Kayser says.
Economics...Colliding With Reality
By late 2013, some regional units of insurance carriers, such as Aetna and Blue Cross, were refusing to pay for proton treatment for prostate cancer.
About 70 percent of all proton therapy patients were receiving treatment for prostate cancer only two years ago, while today only about half of them are prostate patients.
A joint venture involving ProCure, a New Jersey private-equity operator of three proton centers, has struggled to meet targets; at one center, which opened to widespread publicity and investor excitement in 2012, only one-fourth of all patients are coming for prostate cancer treatment, well below the expected 80 percent.
But for young patients like Jonathan, proton therapy does appear to offer more benefit. Conventional therapy, even IMRT, would have irradiated Jonathan’s organs and hip joint, causing acute side effects such as bleeding into the urine, additional diarrhea, rectal bleeding and, soon after, arthritis, likely leading to deformities and disability. Side effects — nausea, infections and radiation injuries — also are more manageable with proton therapy because the beam can avoid sensitive areas from the skin to nearby organs and healthy cells. Simply put, long-term pediatric cancer survivors can be healthier.
Today, Jonathan is learning his times tables and playing soccer. He has regained his balance enough to earn his camouflage belt in tae kwon do. His eyebrows and eyelashes are back. The rosiness has returned to his cheeks.
Jonathan’s doctors say it’s still too early to know the effect of protons on his long-term health. His prognosis is excellent, says Hill-Kayser. There’s no longer a malignant mass, and he hasn’t needed radiation for nearly two years. But cancer can be latent on a microscopic level, so Jonathan returns for scans every four months. “It’s been a shocking, upside-down year, but now we’re really hopeful,” says his mother, Shinna.
She no longer anxiously monitors his intake of meds, supplements and calories lest he lose weight and strength. He sleeps in his own bed again. The darkness in his mother’s spirit has passed, with resumed faith and newfound friends. She no longer asks God, “Why my child?” She marvels at the machines, the know-how, the return to normalcy. “I’ve got my son back,” she says.
[This article originally appeared in print as "Precision vs. Profits."]