Of all the thousands of diseases that keep hypochondriacs awake at night, cancer of the heart is not one of them. Unlike bones, skin, brain, liver, blood, kidneys, and bowels, our heart muscle and the coronary blood vessels that embrace it rarely turn cancerous.
Stephen Epstein knows why cancer of the heart is so rare. As chief of the cardiology branch at the National Heart, Lung, and Blood Institute, Epstein has made it his business to know the heart inside out. Cancer occurs when cells grow out of control, he says. But in the heart and the vessels, cells are not growing very much. The cells divide only maybe once a year. No division, no cancer.
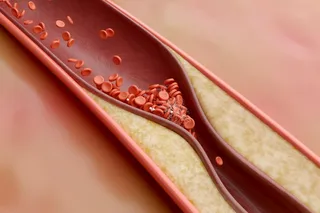
But there is one malady in which cells in the heart’s blood vessels do divide excessively, and its cause is a medical mystery. Every year, some 1 million Americans develop atherosclerosis, a partial or ...